Abstract
BACKGROUND: The anti-CD33 antibody-drug conjugate gemtuzumab ozogamicin (GO) given in combination with intensive chemotherapy has been shown to be active in AML with favorable- and intermediate-risk cytogenetics. AML with mutated NPM1 (NPM1mut) in the majority of cases is associated with normal cytogenetics and with high CD33 expression. Both characteristics provided the rationale for exploring GO in this AML entity. We here present the final results of the phase 3 AMLSG 09-09 trial of intensive chemotherapy with or without GO in patients with newly diagnosed NPM1mut AML(NCT00893399).
METHODS: Patients ≥18 years were randomly assigned to induction therapy with two cycles of idarubicin, cytarabine, etoposide, and all-trans retinoic acid (ATRA) with or without GO. Patients who achieved complete remission (CR) or CR with incomplete hematologic recovery (CRi) received consolidation therapy with 3 cycles of higher doses of cytarabine (age 18-60 years, 3 g/m2 q12 hrs d1-3; age >60 years, 1 g/m2 q12 hrs d1-3) plus ATRA. GO was administered at 3 mg/m2 on d1 of induction cycles 1+2 and of consolidation cycle 1. Randomization was stratified by age (18-60 yrs vs >60 yrs). The two primary endpoints were short-term event-free survival (EFS; 6 months follow-up after inclusion of last patient) and overall survival (OS); secondary endpoints were EFS, rates of CR/CRi, and cumulative incidence of relapse (CIR) and death (CID). Sequential testing was performed for the two primary endpoints with short-term EFS at a significance level of 2% (reported in: Schlenk et al. J Clin Oncol. 2019;38:623-632) and OS at a level of 3%.
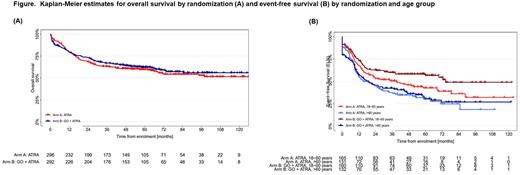
RESULTS: Between May 2010 and September 2017, 588 patients with NPM1mut AML were randomized (standard arm, n=296; GO arm, n=292). Baseline characteristics were balanced between treatment arms: median age 58.8 vs 58.7 yrs; de novo AML 93.2% vs 92.8%; 2010 ELN favorable-risk 73.8% vs 70.7%; FLT3-ITD 16.6% vs 17.1%; DNMT3Amut 41.6% vs 49.5% in the standard and GO arm, respectively; allogeneic hematopoietic-cell transplantation (HCT) in first remission was performed in 9.8% vs 10.6% of the patients. Median follow-up was 64 months. In univariate analysis, there was no difference in OS between treatment arms (hazard ratio [HR] 0.90; 95% confidence interval [CI] 0.70,1.16; stratified logrank test p=0.427). Multivariate Cox regression model using allogeneic HCT as time-dependent variable revealed higher age (HR 1.67; p<0.001), FLT3-ITD (HR 1.94; p<0.001), and DNMT3Amut (HR 1.51; p=0.011) as unfavorable prognostic factors. For the secondary endpoint EFS, univariate analysis showed in trend an advantage for the GO arm (HR 0.83; 95%-CI 0.67,1.03; p=0.078). Multivariate analysis revealed again higher age (HR 1.35; p<0.001), FLT3-ITD (HR 1.40; p=0.032), and DNMT3Amut (HR 1.87; p<0.001) as unfavorable prognostic factors; the HR for treatment with GO was 0.8 (95%-CI 0.64, 1.01; p=0.057). Analysis by age group showed that the effect on EFS was significant in patients 18-60 years of age (HR 0.72; p=0.038), but was not different in patients >60 years (HR 0.95; p=0.659) (Figure below). However, the beneficial effect of GO on EFS in younger patients did not translate into an OS advantage. CIR was significantly in favor of GO (HR 0.65; p=0.003), however, the effect was again restricted to younger patients (18-60 yrs: HR 0.51; p=0.002; >60 yrs: HR 0.80; p=0.265). There was no significant difference in rates of CR/CRi between treatment arms; the 30-day and 60-day mortality was numerically higher in the GO arm (7% vs 4%; and 9% vs 6%, respectively).
CONCLUSIONS: In patients ≥18 years of age with NPM1mut AML, GO in combination with intensive chemotherapy did not improve OS, but in trend had a beneficial effect on EFS. Analysis by age group showed a significant beneficial effect of GO on EFS and CIR in patients 18 to 60 years that however did not translate into an improvement in OS.
Disclosures
Döhner:Kronos Bio, Inc.: Research Funding; Syndax Pharmaceuticals Inc.: Consultancy, Honoraria; Pfizer Inc.: Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Daiichi Sankyo Co, LTD: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Gilead Sciences, Inc.: Consultancy, Honoraria; Berlin-Chemie: Consultancy, Honoraria; AstraZeneca: Honoraria; Brystol Myers Squibb: Consultancy, Honoraria, Research Funding; Astellas Pharma Inc.: Consultancy, Honoraria, Research Funding; Amgen Inc.: Consultancy, Honoraria, Research Funding; Agios Pharmaceuticals: Consultancy, Honoraria, Research Funding; AbbVie Inc.: Consultancy, Honoraria, Research Funding; Janssen Pharmaceuticals: Consultancy, Honoraria; Servier: Consultancy, Honoraria. Kühn:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Other; Gilead: Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Schroeder:Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Mayer:Astellas: Other: Travel suppport; Jazz Pharmaceuticals: Other: Travel suppport; Pfizer: Other: Travel suppport; Novartis: Other: Travel suppport; BMS/Celgene: Other: Travel suppport; Roche: Other: Travel suppport; Amgen: Other: Travel suppport; Teva: Other: Travel suppport. Lübbert:Astex: Honoraria; Janssen: Research Funding; Otsuka: Consultancy; Syros: Consultancy; Cheplapharm: Other: Study drug; Abbvie: Honoraria. Götze:Servier: Honoraria; Abbvie: Honoraria; BMS: Honoraria. Fransecky:Pfizer: Consultancy, Speakers Bureau; Gilead: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau. Koller:Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees. Wulf:Novartis: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau. Schleicher:Apogepha: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria; Ipsen: Honoraria; Pfizer: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; AstraZeneca: Honoraria. Greil:Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Astra Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; MSD Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; BMS-Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Hoffmann - La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding. Martens:BMS: Consultancy, Speakers Bureau; Sanofi: Consultancy. Machherndl-Spandl:Novartis: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Kapp-Schwoerer:Pfizer Inc.: Consultancy; AbbVie Inc.: Consultancy, Honoraria, Other: Travel support; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Travel support. Bullinger:Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer Oncology: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Thol:Takeda: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Heuser:Glycostem: Consultancy, Research Funding; Kura Oncology: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; BMS: Consultancy; Agios: Consultancy, Research Funding; Takeda: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Janssen: Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Eurocept: Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; PinotBio: Consultancy, Research Funding; Tolremo: Consultancy; Astellas: Research Funding; Bayer Pharma AG: Research Funding; BergenBio: Research Funding; Loxo Oncology: Research Funding. Paschka:Abbvie: Consultancy, Other: Travel support; BMS: Consultancy; Pfizer: Speakers Bureau; Janssen: Speakers Bureau. Gaidzik:Abbvie: Honoraria, Speakers Bureau; Janssen: Speakers Bureau; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Pfizer: Speakers Bureau. Schlenk:Pfizer: Honoraria, Research Funding; Abbvie: Research Funding; Novartis: Honoraria; BergenBio: Honoraria; AstraZeneca: Research Funding; PharmaMar: Research Funding; Daiichi Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Döhner:Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Research Funding; Astellas: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kronos: Research Funding. Ganser:Jazz Pharmaceuticals: Consultancy; Novartuis: Consultancy; Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal